amide
Amida structure: R - CONH2
MAKING amide:
Reaction of carboxylic acids with ammonia
Amoniumamida salt is heated
Reaction with acid anhidrid amponiak
USAGE Amida:
A. Formamide is liquid, as solvent.
2. For identification of the liquid acid.
3. For the synthesis of nylon, ds.
Making amides from carboxylic acids Summary of the processThe carboxylic acid is first converted into an ammonium salt which then produces an amide on heating. The ammonium salt is formed by adding solid ammonium carbonate to an excess of the acid. For example, ammonium ethanoate is made by adding ammonium carbonate to an excess of ethanoic acid. When the reaction is complete, the mixture is heated and the ammonium salt dehydrates producing ethanamide. The excess of ethanoic acid is there to prevent dissociation of the ammonium salt before it dehydrates. Ammonium salts tend to split into ammonia and the parent acid on heating, recombining on cooling. If dissociation happened in this case, the ammonia would escape from the reaction mixture and be lost. You couldn't get any recombination. The dissociation is reversible: The presence of the excess ethanoic acid helps to prevent this from happening by moving the position of equilibrium to the left. | |
| | |
| Some brief practical details The ammonium carbonate is added slowly to concentrated ethanoic acid and the reaction is left until all production of carbon dioxide stops. It is then heated under reflux for half an hour for the dehydration to take place. The mixture is distilled at about 170°C to remove excess ethanoic acid and water - leaving almost pure ethanamide in the flask. Further purification stages are beyond the scope of this site. Making amides from acyl chlorides Acyl chlorides (also known as acid chlorides) have the general formula RCOCl. The chlorine atom is very easily replaced by other things. For example, it is easily replaced by an -NH2 group to make an amide. To make ethanamide from ethanoyl chloride, you normally add the ethanoyl chloride to a concentrated solution of ammonia in water. There is a very violent reaction producing lots of white smoke - a mixture of solid ammonium chloride and ethanamide. Some of the mixture remains dissolved in water as a colourless solution. You can think of the reaction as happening in two stages. In the first stage, the ammonia reacts with the ethanoyl chloride to give ethanamide and hydrogen chloride gas. Then the hydrogen chloride produced reacts with excess ammonia to give ammonium chloride. . . . and you can combine all this together to give one overall equation: | |
| | |
| Making amides from acid anhydrides An acid anhydride is what you get if you remove a molecule of water from two carboxylic acid -COOH groups. For example, if you took two ethanoic acid molecules and removed a molecule of water between them you would get the acid anhydride, ethanoic anhydride (old name: acetic anhydride). 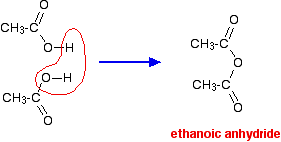 The reactions of acid anhydrides are rather like those of acyl chlorides except that during their reactions, a molecule of carboxylic acid is produced rather than the HCl formed when an acyl chloride reacts. If ethanoic anhydride is added to concentrated ammonia solution, ethanamide is formed together with ammonium ethanoate. Again, the reaction happens in two stages. In the first stage, ethanamide is formed together with ethanoic acid. Then the ethanoic acid produced reacts with excess ammonia to give ammonium ethanoate. . . . and you can combine all this together to give one overall equation: You need to follow this through really carefully, because the two products of the reaction overall can look confusingly similar. | |
Carboxylic Acid
Contains a COOH group attached to the alkyl group (R-COOH) or aryl group (Ar-COOH)
1. Solubility with alcohol
2. Acid by the number of C 1-4: soluble in water
3. Acid by the number of C = 5: poorly soluble in water
4. Acid with a number C> 6: insoluble in water
5. Soluble in organic solvents such as ether, alcohol, and benzene
6.TD carboxylic acids> C TD alcohol with the same amount.
Carboxylic acid structure: R - COOH, and Ar - COOH
CH3-CH2-CH2-CH2-COOH COOH
Valelat
CH3-COOH (acetic acid) benzoic acid
ACID FORMAT = HCOOH
¨ Physical properties: liquid, colorless, damage the skin, smelling, perfectly soluble in H2O.
¨ Use: for the coagulation of latex, penyamakkan leather, textile industry, and fungicides.
ACID-ACETATE = CH 3 COOH
¨ nature: liquid, TL 17oC, 118oC TD, perfectly soluble in H2O
¨ Use: synthesis of acetic acid anhydride, ester, salt, dye, fragrance agents, pharmaceuticals, plastics, artificial fibers, cellulose and as a food additive.
MAKING Carboxylic Acid
Oxidation of primary alcohols
Oxidation of alkyl benzene
Grignard reagent Carbonasi
Hidrolisin nitrile
from your blog,, there "Some brief practical details
BalasHapusThe ammonium carbonate is added slowly to concentrated ethanoic acid and the reaction is left until all production of carbon dioxide stops.
It is then heated under reflux for half an hour for the dehydration to take place.The mixture is distilled at about 170°C", i want ask you, why the temperature at 170°C??why not lower than it? thx