| What are polyamides? Polyamides are polymers where the repeating units are held together by amide links. An amide group has the formula - CONH2. An amide link has this structure: 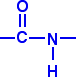 | ||
| | | |
| Nylon In nylon, the repeating units contain chains of carbon atoms. (That is different from Kevlar, where the repeating units contain benzene rings - see below.) There are various different types of nylon depending on the nature of those chains. Nylon-6,6 Nylon-6,6 is made from two monomers each of which contain 6 carbon atoms - hence its name. One of the monomers is a 6 carbon acid with a -COOH group at each end - hexanedioic acid. | ||
| . | ||
| The other monomer is a 6 carbon chain with an amino group, -NH2, at each end. This is 1,6-diaminohexane (also known as hexane-1,6-diamine). Condensation polymerisation is the formation of a polymer involving the loss of a small molecule. In this case, the molecule is water, but in other cases different small molecules might be lost. The diagram shows the loss of water between two of the monomers: 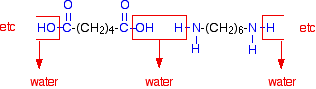 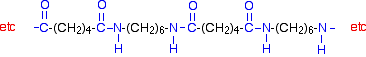 | ||
| Nylon-6 If you are doing UK A level, you are unlikely to need the structure of nylon-6. I am including it to show that it is possible to get a polyamide from a single monomer. Nylon-6 is made from a monomer called caprolactam. 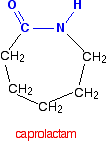 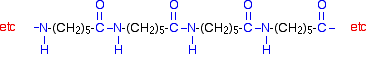 Kevlar is similar in structure to nylon-6,6 except that instead of the amide links joining chains of carbon atoms together, they join benzene rings. The two monomers are benzene-1,4-dicarboxylic acid and 1,4-diaminobenzene. 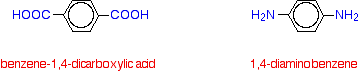 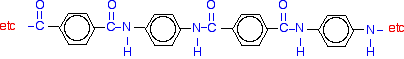 Nylon-6,6 is made by polymerising hexanedioic acid and 1,6-diaminohexane exactly as shown further up the page. Because the acid is acidic and the amine is basic, they first react together to form a salt. That is then converted into nylon-6,6 by heating it under pressure at 350°C. The two monomers can both be made from cyclohexane.
In the lab, it is easy to make nylon-6,6 at room temperature using an acyl chloride (acid chloride) rather than an acid. The 1,6-diaminohexane is used just as before, but hexanedioyl dichloride is used instead of hexanedioic acid. 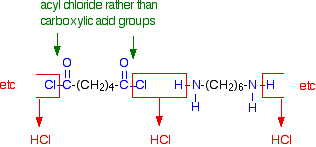 | ||
| | ||
| In the lab, this reaction is the basis for the nylon rope trick. You make a solution of the hexanedioyl dichloride in an organic solvent, and a solution of 1,6-diaminohexane in water. You carefully float one solution on top of the other in a small beaker, taking care to get as little mixing as possible. Nylon-6,6 forms at the boundary between the two solutions. If you pick up the boundary layer with a pair of tweezers, you can pull out an amazingly long tube of nylon from the beaker. | ||
| Hydrolysis of polyamides Simple amides are easily hydrolysed by reaction with dilute acids or alkalis. Polyamides are fairly readily attacked by strong acids, but are much more resistant to alkaline hydrolysis. Hydrolysis is faster at higher temperatures. Hydrolysis by water alone is so slow as to be completely unimportant. Kevlar is rather more resistant to hydrolysis than nylon is. If you spill something like dilute sulphuric acid on a fabric made from nylon, the amide linkages are broken. The long chains break and you can eventually end up with the original monomers - hexanedioic acid and 1,6-diaminohexane. Because you produce small molecules rather than the original polymer, the fibres are destroyed, and you end up with a hole! | ||
| Uses of polyamides Nylon Apart from obvious uses in textiles for clothing and carpets, a lot of nylon is used to make tyre cords - the inner structure of a vehicle tyre underneath the rubber. The fibres are also used in ropes, and nylon can be cast into solid shapes for cogs and bearings in machines, for example. Kevlar Kevlar is a very strong material - about five times as strong as steel, weight for weight. It is used in bulletproof vests, in composites for boat construction, in lightweight mountaineering ropes, and for lightweight skis and racquets - amongst many other things. | ||
Kamis, 31 Mei 2012
"POLYAMIDES"
"The hydrolysis of amides"
| The hydrolysis of amides What is hydrolysis? Technically, hydrolysis is a reaction with water. That is exactly what happens when amides are hydrolysed in the presence of dilute acids such as dilute hydrochloric acid. The acid acts as a catalyst for the reaction between the amide and water. The alkaline hydrolysis of amides actually involves reaction with hydroxide ions, but the result is similar enough that it is still classed as hydrolysis. Hydrolysis under acidic conditions Taking ethanamide as a typical amide: If ethanamide is heated with a dilute acid (such as dilute hydrochloric acid), ethanoic acid is formed together with ammonium ions. So, if you were using hydrochloric acid, the final solution would contain ammonium chloride and ethanoic acid. | ||
| | ||
| Hydrolysis under alkaline conditions Again, taking ethanamide as a typical amide: If ethanamide is heated with sodium hydroxide solution, ammonia gas is given off and you are left with a solution containing sodium ethanoate. Using alkaline hydrolysis to test for an amide If you add sodium hydroxide solution to an unknown organic compound, and it gives off ammonia on heating (but not immediately in the cold), then it is an amide. You can recognise the ammonia by smell and because it turns red litmus paper blue. The possible confusion using this test is with ammonium salts. Ammonium salts also produce ammonia with sodium hydroxide solution, but in this case there is always enough ammonia produced in the cold for the smell to be immediately obvious. | ||
| | ||
introducing amides
| INTRODUCING AMIDES . What are amides? Amides are derived from carboxylic acids. A carboxylic acid contains the -COOH group, and in an amide the -OH part of that group is replaced by an -NH2 group. So . . . amides contain the -CONH2 group. Some simple amides The most commonly discussed amide is ethanamide, CH3CONH2 (old name: acetamide). 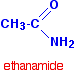
If the chain was branched, the carbon in the -CONH2 group counts as the number 1 carbon atom. For example: 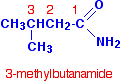 Physical properties Melting points Methanamide is a liquid at room temperature (melting point: 3°C), but the other amides are solid. For example, ethanamide forms colourless deliquescent crystals with a melting point of 82°C. A deliquescent substance is one which picks up water from the atmosphere and dissolves in it. Ethanamide crystals nearly always look wet. | |||||||
The melting points of the amides are high for the size of the molecules because they can form hydrogen bonds. The hydrogen atoms in the -NH2 group are sufficiently positive to form a hydrogen bond with a lone pair on the oxygen atom of another molecule.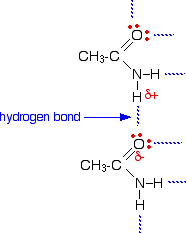 These hydrogen bonds need a reasonable amount of energy to break, and so the melting points of the amides are quite high. | |||||||
| | |||||||
| Solubility in water The small amides are soluble in water because they have the ability to hydrogen bond with the water molecules. It needs energy to break the hydrogen bonds between amide molecules and between water molecules before they can mix - but enough energy is released again when the new hydrogen bonds are set up to allow this to happen. | |||||||
"Making amide"
amide
Amida structure: R - CONH2
MAKING amide:
Reaction of carboxylic acids with ammonia
Amoniumamida salt is heated
Reaction with acid anhidrid amponiak
USAGE Amida:
A. Formamide is liquid, as solvent.
2. For identification of the liquid acid.
3. For the synthesis of nylon, ds.
Making amides from carboxylic acids Summary of the processThe carboxylic acid is first converted into an ammonium salt which then produces an amide on heating. The ammonium salt is formed by adding solid ammonium carbonate to an excess of the acid. For example, ammonium ethanoate is made by adding ammonium carbonate to an excess of ethanoic acid. When the reaction is complete, the mixture is heated and the ammonium salt dehydrates producing ethanamide. The excess of ethanoic acid is there to prevent dissociation of the ammonium salt before it dehydrates. Ammonium salts tend to split into ammonia and the parent acid on heating, recombining on cooling. If dissociation happened in this case, the ammonia would escape from the reaction mixture and be lost. You couldn't get any recombination. The dissociation is reversible: The presence of the excess ethanoic acid helps to prevent this from happening by moving the position of equilibrium to the left. | |
| | |
| Some brief practical details The ammonium carbonate is added slowly to concentrated ethanoic acid and the reaction is left until all production of carbon dioxide stops. It is then heated under reflux for half an hour for the dehydration to take place. The mixture is distilled at about 170°C to remove excess ethanoic acid and water - leaving almost pure ethanamide in the flask. Further purification stages are beyond the scope of this site. Making amides from acyl chlorides Acyl chlorides (also known as acid chlorides) have the general formula RCOCl. The chlorine atom is very easily replaced by other things. For example, it is easily replaced by an -NH2 group to make an amide. To make ethanamide from ethanoyl chloride, you normally add the ethanoyl chloride to a concentrated solution of ammonia in water. There is a very violent reaction producing lots of white smoke - a mixture of solid ammonium chloride and ethanamide. Some of the mixture remains dissolved in water as a colourless solution. You can think of the reaction as happening in two stages. In the first stage, the ammonia reacts with the ethanoyl chloride to give ethanamide and hydrogen chloride gas. Then the hydrogen chloride produced reacts with excess ammonia to give ammonium chloride. . . . and you can combine all this together to give one overall equation: | |
| | |
| Making amides from acid anhydrides An acid anhydride is what you get if you remove a molecule of water from two carboxylic acid -COOH groups. For example, if you took two ethanoic acid molecules and removed a molecule of water between them you would get the acid anhydride, ethanoic anhydride (old name: acetic anhydride). 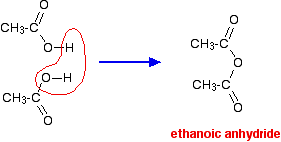 The reactions of acid anhydrides are rather like those of acyl chlorides except that during their reactions, a molecule of carboxylic acid is produced rather than the HCl formed when an acyl chloride reacts. If ethanoic anhydride is added to concentrated ammonia solution, ethanamide is formed together with ammonium ethanoate. Again, the reaction happens in two stages. In the first stage, ethanamide is formed together with ethanoic acid. Then the ethanoic acid produced reacts with excess ammonia to give ammonium ethanoate. . . . and you can combine all this together to give one overall equation: You need to follow this through really carefully, because the two products of the reaction overall can look confusingly similar. | |
Carboxylic Acid
Contains a COOH group attached to the alkyl group (R-COOH) or aryl group (Ar-COOH)
1. Solubility with alcohol
2. Acid by the number of C 1-4: soluble in water
3. Acid by the number of C = 5: poorly soluble in water
4. Acid with a number C> 6: insoluble in water
5. Soluble in organic solvents such as ether, alcohol, and benzene
6.TD carboxylic acids> C TD alcohol with the same amount.
Carboxylic acid structure: R - COOH, and Ar - COOH
CH3-CH2-CH2-CH2-COOH COOH
Valelat
CH3-COOH (acetic acid) benzoic acid
ACID FORMAT = HCOOH
¨ Physical properties: liquid, colorless, damage the skin, smelling, perfectly soluble in H2O.
¨ Use: for the coagulation of latex, penyamakkan leather, textile industry, and fungicides.
ACID-ACETATE = CH 3 COOH
¨ nature: liquid, TL 17oC, 118oC TD, perfectly soluble in H2O
¨ Use: synthesis of acetic acid anhydride, ester, salt, dye, fragrance agents, pharmaceuticals, plastics, artificial fibers, cellulose and as a food additive.
MAKING Carboxylic Acid
Oxidation of primary alcohols
Oxidation of alkyl benzene
Grignard reagent Carbonasi
Hidrolisin nitrile
amide
AMIDE
A. Introduction
Judging from its structure carboxylic acid derivatives are compounds derived from the turn of the-OH group in the structure of the formula RC-OOH by the group X (halogen),-NH 2 OR ', or-OOCR. Each replacement of an acyl group of compounds of different nature and a row is called the acid halide (R-COX), amides (RCONH2) ester (RCOOR '), and carboxylic acid anhydride (RCOOORCR).
As well as carboxylic acids, acid derivatives karbosilat also differentiated into derivatives of aliphatic or aromatic carboxylic acids, both substituted maupaun are not substituted. All carboxylic acid derivatives having acyl functional group (RCO-) or aroil (ARCO-) and when hydrolyzed produce carboxylic acids. Side results in the hydrolysis of the type of acid derivative tergatung karboksilatnya.
The existence of the carbonyl group in carboxylic acid derivatives led to the molecule is polar. This polarity which affects the physical properties and chemical carboxylic acid derivatives.
2. Nomenclature
Amide is a compound having a trivalent nitrogen bonded to a carbonyl group. In the amide compound, gugusfungsi acyl-NH2 group relates to. In giving his name, the suffix-At-Oat or in the name of the parent acid is replaced with an amide.
Example:
1. HCOOH: metanoat acid / formic acid
2. HCONH2: metanamida (IUPAC)
Formamide (nontrivial)
3. CH3CH2CH2COOH: bityanoat acid / butyric acid
4. CH3CH2CH2CONH2: butanamida (IUPAC)
Butiramida (nontrivial)
3. Physical properties Sifar
Polar molecules of carboxylic acid derivatives of compounds caused by adanaya carbonyl group (-C-), affects the physical properties (boiling point, melting point and solubility) note that titij boiling acid halide, carboxylic acid anhydride and the ester is almost the same almost the same with aldehydes and ketones boiling titk a comparable molecular brat. Keep in mind that the aldehydes and ketones are compounds which also contain carbonyl groups. Especially for the amide compound, the price was high enough boiling point. This is caused by inter-molecular hydrogen adanyai Katan described as follows:
R
H C
O ... N - H .... O N - H
C H
R
All dapay carboxylic acid derivatives soluble in organic solvents, whereas the water solubility depending on the number of carbon atoms contained in the molecule. For example, for the ester group of compounds containing 3-5 C atoms are soluble in water, but for the amide group of compounds that are soluble in water which has a 5-6 atom C.
Various volatile esters have pleasant odors that are often used in the manufacture of perfumes or synthetic flavoring ingredient. Acid chloride group of compounds having a sharp odor because it is easily hydrolyzed and produce carboxylic acid and HCL which each have a distinctive odor.
4. Chemical properties
In studying the chemical properties of each group of carboxylic acid derivatives, it must first be understood. General characteristics of the reaction as described below:
a. The presence of carbonyl group in carboxylic acid derivatives determine the reactivity of the reaction, although the carbonyl group is not changed.
b. Acyl group (RC = O), causing carboxylic acid derivatives susceptible to nucleophilic substitution. In this substitution, atom / group associated with the acyl group was replaced by another group which is alkaline. General pattern of nucleophilic substitution reactions are written by the equation
c. Nucleophilic substitution reactions on carboxylic acid derivatives is faster than the nucleophilic substitution reactions at saturated carbon chain (alkyl group),
5. Description of Amida
Amide is a compound that is karbosilat acid derivatives obtained from the replacement of-OH to-COOH group by group-NH2.
6. Used amide
Amide prepared by reacting ammonia on the acid chloride or acid anhydride, while the industry is made by heating ammonium carboxylate salts.
amide
Amide refers to compounds with the functional group RnE(O)xNR'2 (R and R' refer to H or organic groups). Most common are "organic amides" (n = 1, E = C, x = 1), but many other important types of amides are known including phosphor amides (n = 2, E = P, x = 1 and many related formulas) and sulfonamides (E = S, x= 2). The term amide refers both to classes of compounds and to the functional group (RnE(O)xNR'2) within those compounds.
Amide can also refer to a conjugate base of ammonia and an organic amine, represented as anions R2N–. For discussion of these "anionic amides," see the articles sodium amide and lithium diisopropylamide.
Structure and bonding
The simplest amides are derivatives of ammonia wherein one hydrogen atom has been replaced by an acyl group. The ensemble is generally represented as RC(O)NH2. Closely related and even more numerous are amides derived from primary amines (R'NH2) with the formula RC(O)NHR'. Amides are also commonly derived from secondary amines (R'RNH) with the formula RC(O)NR'R. Amide are usually regarded as derivatives of carboxylic acids in which the hydroxyl group has been replaced by an amine or ammonia.
The simplest amides are derivatives of ammonia wherein one hydrogen atom has been replaced by an acyl group. The ensemble is generally represented as RC(O)NH2. Closely related and even more numerous are amides derived from primary amines (R'NH2) with the formula RC(O)NHR'. Amides are also commonly derived from secondary amines (R'RNH) with the formula RC(O)NR'R. Amide are usually regarded as derivatives of carboxylic acids in which the hydroxyl group has been replaced by an amine or ammonia.
Some chemists make a pronunciation distinction between the two, saying /əˈmiːd/ for the carbonyl-nitrogen compound and i/ˈæmaɪd/ for the anion. Others substitute one of these with /ˈæmɨd/, while still others pronounce both /ˈæmɨd/, making them homonyms.
Langganan:
Postingan (Atom)