What are nitriles? Nitriles contain the -CN group, and used to be known as cyanides.
Nitriles
Nomenclature
| Formula | 3D structure |
Functional class = alkyl cyanide
Functional group suffix = nitrile or -onitrile
Substituent prefix = cyano-
Notes :
- The cyano prefix is used in a very similar manner to haloalkanes
- The cyano nomenclature is most common when the alkyl group is simple.
- The nitrile suffix is used in a very similar manner to carboxylic acids.
| 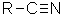 | |
Cyano substituent style:
- The root name is based on the longest chain with the -C≡N as a substituent.
- This root give the alkane part of the name.
- The chain is numbered so as to give the -C≡N group the lowest possible locant number
Nitrile style:
- The root name is based on the longest chain including the carbon of the nitrile group.
- This root give the alkyl part of the name.
- Since the nitrile must be at the end of the chain, it must be C1 and no locant needs to be specified.
- Nitriles can also be named by replacing the -oic acid suffix of the corresponding carboxylic acid with -onitrile.
Cyano substituent style: - Functional group is an alkane, therefore suffix = -ane
- The longest continuous chain is C3 therefore root = prop
- The substituent is a -CN therefore prefix = cyano
- The first point of difference rule requires numbering from the right as drawn, the substituent locant is 1-
1-cyanopropane
Nitrile style:
- Functional group is a -C≡N, therefore suffix = -nitrile
- Hydrocarbon structure is an alkane therefore -ane
- The longest continuous chain is C4 therefore root = but
butanenitrile |
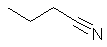
CH3CH2CH2C≡N |
|
Some simple nitriles
The smallest organic nitrile is ethanenitrile, CH3CN, (old name: methyl cyanide or acetonitrile - and sometimes now called ethanonitrile). Hydrogen cyanide, HCN, doesn't usually count as organic, even though it contains a carbon atom.
Notice the triple bond between the carbon and nitrogen in the -CN group.
The three simplest nitriles are:
| CH3CN | ethanenitrile |
| CH3CH2CN | propanenitrile |
| CH3CH2CH2CN | butanenitrile |
When you are counting the length of the carbon chain, don't forget the carbon in the -CN group. If the chain is branched, this carbon usually counts as the number 1 carbon.
|
Solubility in water
Ethanenitrile is completely soluble in water, and the solubility then falls as chain length increases.
| nitrile | solubility at 20°C |
|---|
| CH3CN | miscible |
| CH3CH2CN | 10 g per 100 cm3 of water |
| CH3CH2CH2CN | 3 g per 100 cm3 of water |
The reason for the solubility is that although nitriles can't hydrogen bond with themselves, they can hydrogen bond with water molecules.
One of the slightly positive hydrogen atoms in a water molecule is attracted to the lone pair on the nitrogen atom in a nitrile and a hydrogen bond is formed.
There will also, of course, be dispersion forces and dipole-dipole attractions between the nitrile and water molecules.
Forming these attractions releases energy. This helps to supply the energy needed to separate water molecule from water molecule and nitrile molecule from nitrile molecule before they can mix together.
As chain lengths increase, the hydrocarbon parts of the nitrile molecules start to get in the way.
By forcing themselves between water molecules, they break the relatively strong hydrogen bonds between water molecules without replacing them by anything as good. This makes the process energetically less profitable, and so solubility decreases.
Making nitriles
making nitriles from halogenoalkanes The halogenoalkane is heated under reflux with a solution of sodium or potassium cyanide in ethanol. The halogen is replaced by a -CN group and a nitrile is produced. Heating under reflux means heating with a condenser placed vertically in the flask to prevent loss of volatile substances from the mixture.
The solvent is important. If water is present you tend to get substitution by -OH instead of -CN.
|
| |
For example, using 1-bromopropane as a typical halogenoalkane:
 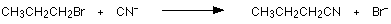
You could write the full equation rather than the ionic one, but it slightly obscures what's going on:
 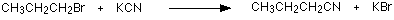
The bromine (or other halogen) in the halogenoalkane is simply replaced by a -CN group - hence a substitution reaction. In this example, butanenitrile is formed.
|
| |
Making a nitrile by this method is a useful way of increasing the length of a carbon chain. Having made the nitrile, the -CN group can easily be modified to make other things - as you will find if you explore the nitriles menu.
Making nitriles from amides Nitriles can be made by dehydrating amides.
Amides are dehydrated by heating a solid mixture of the amide and phosphorus(V) oxide, P4O10.
Water is removed from the amide group to leave a nitrile group, -CN. The liquid nitrile is collected by simple distillation.
For example, you will get ethanenitrile by dehydrating ethanamide.
 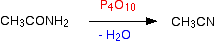
|
| |
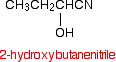
Making nitriles from aldehydes and ketones Aldehydes and ketones undergo an addition reaction with hydrogen cyanide. The hydrogen cyanide adds across the carbon-oxygen double bond in the aldehyde or ketone to produce a hydroxynitrile. Hydroxynitriles used to be known as cyanohydrins.
For example, with ethanal (an aldehyde) you get 2-hydroxypropanenitrile:
 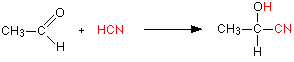
With propanone (a ketone) you get 2-hydroxy-2-methylpropanenitrile:
 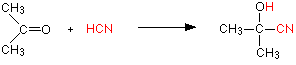
In every example of this kind, the -OH group will be on the number 2 carbon atom - the one next to the -CN group.
The reaction isn't normally done using hydrogen cyanide itself, because this is an extremely poisonous gas. Instead, the aldehyde or ketone is mixed with a solution of sodium or potassium cyanide in water to which a little sulphuric acid has been added. The pH of the solution is adjusted to about 4 - 5, because this gives the fastest reaction. The reaction happens at room temperature.
The solution will contain hydrogen cyanide (from the reaction between the sodium or potassium cyanide and the sulphuric acid), but still contains some free cyanide ions. This is important for the mechanism.
|
| |
These are useful reactions because they not only increase the number of carbon atoms in a chain, but also introduce another reactive group as well as the -CN group. The -OH group behaves just like the -OH group in any alcohol with a similar structure.
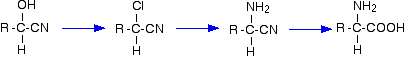
For example, starting from a hydroxynitrile made from an aldehyde, you can quite easily produce relatively complicated molecules like 2-amino acids - the amino acids which are used to construct proteins.
|
|
|
|
The hydrolysis of nitriles
Introduction
When nitriles are hydrolysed you can think of them reacting with water in two stages - first to produce an amide, and then the ammonium salt of a carboxylic acid.
For example, ethanenitrile would end up as ammonium ethanoate going via ethanamide.
 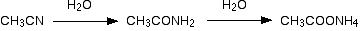
In practice, the reaction between nitriles and water would be so slow as to be completely negligible. The nitrile is instead heated with either a dilute acid such as dilute hydrochloric acid, or with an alkali such as sodium hydroxide solution.
The end result is similar in all the cases, but the exact nature of the final product varies depending on the conditions you use for the reaction.
Acidic hydrolysis of nitriles
The nitrile is heated under reflux with dilute hydrochloric acid. Instead of getting an ammonium salt as you would do if the reaction only involved water, you produce the free carboxylic acid.
For example, with ethanenitrile and hydrochloric acid you would get ethanoic acid and ammonium chloride.
 
Why is the free acid formed rather than the ammonium salt? The ethanoate ions in the ammonium ethanoate react with hydrogen ions from the hydrochloric acid to produce ethanoic acid. Ethanoic acid is only a weak acid and so once it has got the hydrogen ion, it tends to hang on to it.
Alkaline hydrolysis of nitriles
The nitrile is heated under reflux with sodium hydroxide solution. This time, instead of getting an ammonium salt as you would do if the reaction only involved water, you get the sodium salt. Ammonia gas is given off as well.
For example, with ethanenitrile and sodium hydroxide solution you would get sodium ethanoate and ammonia.
 
The ammonia is formed from reaction between ammonium ions and hydroxide ions.
If you wanted the free carboxylic acid in this case, you would have to acidify the final solution with a strong acid such as dilute hydrochloric acid or dilute sulphuric acid. The ethanoate ion in the sodium ethanoate will react with hydrogen ions as mentioned above.
|
|
|
The reduction of nitriles using LiAlH4
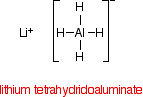
The reducing agent
Despite its name, the structure of the reducing agent is very simple. There are four hydrogens ("tetrahydido") around the aluminium in a negative ion (shown by the "ate" ending).
The "(III)" shows the oxidation state of the aluminium, and is often left out because aluminium only ever shows the +3 oxidation state in its compounds. To make the name shorter, that's what I shall do for the rest of this page.
|
| |
The structure of LiAlH4 is:
In the negative ion, one of the bonds is a co-ordinate covalent (dative covalent) bond using the lone pair on a hydride ion (H-) to form a bond with an empty orbital on the aluminium.
|
| |
The overall reaction
The nitrile reacts with the lithium tetrahydridoaluminate in solution in ethoxyethane (diethyl ether, or just "ether") followed by treatment of the product of that reaction with a dilute acid.
Overall, the carbon-nitrogen triple bond is reduced to give a primary amine. Primary amines contain the -NH2 group.
For example, with ethanenitrile you get ethylamine:
 
Notice that this is a simplified equation - perfectly acceptable to UK A level examiners. [H] means "hydrogen from a reducing agent".
|
| |
The reduction of nitriles using hydrogen and a metal catalyst
The carbon-nitrogen triple bond in a nitrile can also be reduced by reaction with hydrogen gas in the presence of a variety of metal catalysts.
Commonly quoted catalysts are palladium, platinum or nickel.
The reaction will take place at a raised temperature and pressure. It is impossible to give exact details because it will vary from catalyst to catalyst.
For example, ethanenitrile can be reduced to ethylamine by reaction with hydrogen in the presence of a palladium catalyst.
 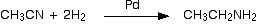
|
|
|
|
|
|
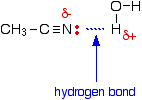
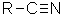

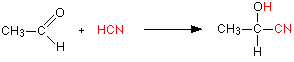
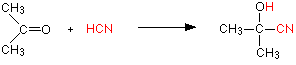
Good!!!but i still confused what is purpose why did we hydrolyze nitriles? And how important Nitriles in our life? Thanks so much CT
BalasHapusthx via,,
BalasHapusthe hydroliza nitriles to know reacting with water in two stages - first to produce an amide, and then the ammonium salt of a carboxylic acid.
For example, ethanenitrile would end up as ammonium ethanoate going via ethanamide.
In practice, the reaction between nitriles and water would be so slow as to be completely negligible. The nitrile is instead heated with either a dilute acid such as dilute hydrochloric acid, or with an alkali such as sodium hydroxide solution.
one in another Function of nitrite is to stabilize the color of the network to contribute to the character of the meat curing to inhibit the growth of food poisoning and spoilage microorganisms, inhibit rancidity.
Pauline
BalasHapusI read the article, there should be formed nitrite in the stomach. is it true nitrite can be formed in the human stomach is a lot nitrates found in vegetables, with the help of bacteria???
why the carbon-nitrogen triple bond in a nitrile, commonly quoted catalysts are palladium, platinum or nickel? why isn't other metal?
BalasHapusWhy R at the R-CN structure influenced boiling points at the nitriles?.
BalasHapusthx miss olin..
BalasHapusits true miss olin,, because The use of nitrite and nitrate in food and the other is limited because there are toxic effects of these substances. Nitrite reacts with the amino secondary / tertiary form the compound N-nitrosamines which are mutagens and carcinogens, further carcinogenic nitrosamines showed activity. Nitrite residues are left in the final product will cause death if more than 15-20mg / kg body weight is consumed.
thx miss yuli,,, ^__^
BalasHapusi will try to answer your question.
The carbon-nitrogen triple bond in a nitrile can also be reduced by reaction with hydrogen gas in the presence of a variety of metal catalysts.
Commonly quoted catalysts are palladium, platinum or nickel.
The reaction will take place at a raised temperature and pressure. It is impossible to give exact details because it will vary from catalyst to catalyst.
For example, ethanenitrile can be reduced to ethylamine by reaction with hydrogen in the presence of a palladium catalyst.