Stereochemistry is another name for the optical activity that a molecule expresses. Optical activity is a property of organic molecules So far several methods have been presented to distinguish between organic molecules. They can be classified as alkanes, alkenes, or alkynes depending on the presence of double and triple bonds, and their functional groups can be identified. A systematic method for naming compounds has also been presented, allowing different compounds to be differentiated.
Somtimes, two different molecules can possess the same chemical formula. Two distinct molecules with the same chemical formula are called isomers.
Constitutional isomers
Constitutational isomers or structural isomers are molecules with the same chemical formula but different structures of atoms and bonds. For example, both 3-methylpentane and hexane have the same chemical formula, C6H14, yet they clearly have different structures: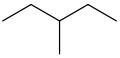 | 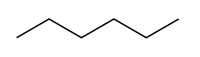 |
| 3-methylpentane | hexane |
Another example involves functional groups. Methoxy methane, an ether, and ethanol, an alcohol, both have the chemical formula C2H6O:
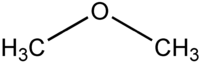 | 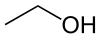 |
| Methoxy methane | Ethanol |
Enantiomers
Sometimes, two molecules can have the same chemical formula and even the same structure of atoms bonded to each other but can still be distinct molecules (isomers). How can this be so? Not only the arrangement in which atoms are bonded to each other, but also the actual geometry of those bonds in space, determine the properties of a molecule. Stereoisomers are distinct molecules with the same sequence of bonded atoms but a different orientation of these atoms in space. Consider the following representations of the molecule bromochlorofluromethane: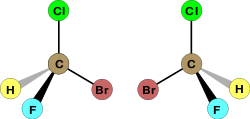
| S enantiomer | R enantiomer |
The two representations clearly depict the same structure of atoms bonded to each other. Yet they are clearly different molecules. No rotation will cause the molecule on the right to adopt the same arrangement of bonds in space as the molecule on the left. If bromine is rotated to adopt the same location as the molecule on the left, then the hydrogen and fluorine atoms will have switched places. Clearly the two molecules are different. In fact they are mirror images of each other. What is special about bromochlorofluromethane is that its mirror image can never be manipulated to produce the original molecule; that is, the mirror image can never be superimposed on the original. Two molecules which are non-superimposibile mirror images of each other are called enantiomers. One special property of enantiomers is that despite being distinct molecules, enantiomers have the same physical properties. The only exception is that enantiomers will rotate plane polarized light in different directions. This property is used to distinguish them. The amino acids that compose proteins in living organisms are enantiomers, but only one form exists in most living organisms. Thus, often only one enantiomer of a drug molecule will have an effect in the human body.
The simplest example of enantiomers involve four different groups bonded to a central carbon atom. When the four groups are distinct, the molecule is an enantiomer, and the carbon atom is called a chiral center or stereogenic center. Two enantiomers can be distinguished by assigning a letter to the chiral center, either R or S. First, the three substituents are numbered according to atomic mass. The atom with the highest atomic mass is assigned highest priority (1), the second heaviest is assigned next highest priority (2), and so on. If two atoms are the same, the atoms bonded to them are examined, and so on until the first point of difference, at which point the assignment of priority is made. If a rotation through the numbers 1, 2, and 3 is clockwise, the enantiomer is labeled R; if it is counterclockwise, it is labeled S. Let's assign R or S configurations to the above molecules. In the molecules above, bromine is the heaviest, so it get the highest priority (1). Chlorine comes next (2). Flourine comes third (3). In the molecule on the left, if we follow along passing from 1 to 2 to 3, we find that we have made a rotation in the counterclockwise direction; thus the left molecule is the S enantiomer. If we now follow from 1 to 2 to 3 on the right, we find that we have moved in a clockwise direction; the molecule on the right is the R enantiomer. The system of assigning R or S labels to enantiomers, called the Cahn–Ingold–Prelog system is a simple way of distinguishing between enantiomers.
Diastereomers
Diastereomers are stereoisomers that are not enantiomers; that is, they are distinct molecules with the same structural arrangement of atoms that are non-superimposible, non-mirror images of each other. Consider the following representations of tartaric acid: |  | 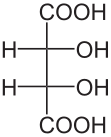 |
| (2S,3S)-tartaric acid | (2R,3R)-tartaric acid | (2R,3S)-tartaric acid |
In tartaric acid, both of the central carbon atoms are stereogenic centers. Thus, they both must be assigned R or S configurations according to the Cahn–Ingold–Prelog system. The first two molecules shown above, (2S,3S)-tartaric acid and (2R,3R)-tartaric acid, are clearly enantiomers of each other since they are mirror images. But what about the third molecule, (2R,3S)-tartaric acid? It is clearly distinct from the first two, yet the same atoms are bonded to each other. It is also very clearly not a mirror image of either of the first two. Thus, as a non-superimposible, non-mirror image with the same arrangement of atoms, (2R,3S)-tartaric acid is a diasteromer of the first two molecules.
Another type of diastereomer can occur in the presence of a double bond. Consider the following representations of the molecule 2-butene:
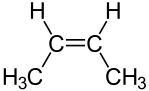 | 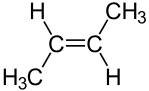 |
| cis-2-butene | trans-2-butene |
The molecules are clearly distinct; they clearly have the same arrangement of atoms structurally; and they clearly are not mirror images. Thus, these molecules are diastereomers. Diastereomers such as these can be distinguished using the Cahn–Ingold–Prelog system. If there are only two substituents, as above, the molecule is simply inspected to determine whether the higher priority substituents are on the same side of the double bond. If they are, the molecule is designated cis. If not, it is designated trans. If more than two different substituents are present on the double bond, then the highest priority substitunet on each carbon atom composing the double bond is identified. If these highest priority substituents are on the same of the double bond, the molecule is designated Z; if not, it is designated E.
Tidak ada komentar:
Posting Komentar