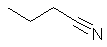
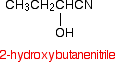
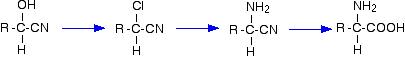
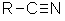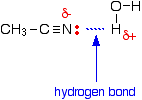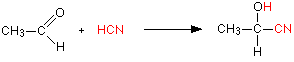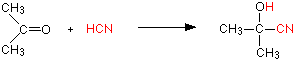Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules.
Stereochemistry is also known as 3D chemistry because the prefix "stereo-" means "three-dimensionality".
The study of stereochemical problems spans the entire range of organic, inorganic, biological, physical and supramolecular chemistries. Stereochemistry includes methods for determining and describing these relationships; the effect on the physical or biological properties these relationships impart upon the molecules in question, and the manner in which these relationships influence the reactivity of the molecules in question (dynamic stereochemistry).
Structural diagrams which depict stereochemistry must be prepared with extra care to ensure there is no ambiguity. The ability to proficiently draw and read such structures requires some practice with reference to 3D molecular models. Some simple "do's" and "don'ts" of the art of stereochemical drawing are illustrated below. In general, the molecules are presented in some kind of perspective drawing, based on the idea that the four substituents of a tetrahedral center can be divided into two pairs, laying in mutually perpendicular planes. Most often the center and two of such substituents are shown in the plane of the drawing (i.e. the plane of the drawing surface) and their bonds are depicted as plain lines (  ).
).
Bonds to the other two substituents are shown with different symbols. Bonds to atoms above the plane of the drawing (coming out, toward the viewer) are shown with a bold wedge ( ), with the narrow end of the wedge starting at the stereogenic center. As an alternative bald bar bonds (
), with the narrow end of the wedge starting at the stereogenic center. As an alternative bald bar bonds (  ) are used.
) are used.
Bonds to atoms below the plane (going in, away from the viewer) are shown with hash wedges ( ). There are two separate conventions in use. In the American usage the narrow edge points to the central atom, while in the European convention, the wide edge points to the central atom. As an alternative, a bar of hash lines (
). There are two separate conventions in use. In the American usage the narrow edge points to the central atom, while in the European convention, the wide edge points to the central atom. As an alternative, a bar of hash lines (  ) is used. A broken line (
) is used. A broken line ( ) or an open wedge (
) or an open wedge (  ) can also be found in some drawings, but their usage is discouraged.
) can also be found in some drawings, but their usage is discouraged.
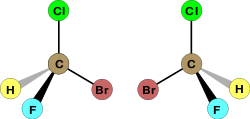
These various presentations are illustrated below on an example of a compound with one stereocenter (in all cases, except where indicated, the same S absolute stereochemistry is shown). The first structure (A) is the favored presentation, while B shows a rotational variant of A (any rotation within the plane of these drawings is perfectly acceptable). Structure C demonstrates the European convention that has a more consistent perspective view of the wedges. Structure D shows the use of open wedge (not recommended), while structure E illustrates the use of bar bonds, that are commonly employed to designate relative stereochemistry. In our class we will use wedges (A) to indicate absolute stereochemistry (one enantiomer), and bars (E) to designate racemic mixtures (and relative stereochemistry, see below).
In addition to structures with two bonds to the stereocenter in the plane of the drawing (above), some representations may have only one bond in that plane (F and G) and some have none (H and I), as shown below. In structures like F and G or H and I, three or four wedged lines are used to designate the 3D disposition of substituents, respectively.
Confusingly, one may often encounter other 3D structures that seem to follow the above conventions, but are in fact different perspective representations. In structures J–M the plain bonds (–––) are not in the plane of the drawing. Their 3D disposition is implied by the wedge lines present. For example, in structure J the wedge bond is a mast pole on three legs, and in L and M the plain-line bonds are really going in or out of the plane of the drawing, respectively..
All these arrangements may be visualized in 3D by selecting the appropriate radio-button in the Jmol applet. Of course C, D, and E are in the exact same orientation as A and are not repeated. Note that all the manipulations are just simple rotations of the same molecule.
Paying attention to the correct representation of the perspective is crucial. Some incorrect usage of wedge bonds is illustrated in structures N and O. Despite appearances, there is no stereochemical information in such structures. A couple of simple rules can be used to decide adequacy of such drawings: (1) the bold-wedge and the hash-wedge substituents must be on the same side of an imaginary line connecting the plain-line bonded substituents (as in structures A or B), and (2) two bold-wedge subsituents (or two hash-wedge substituents) must be on the opposite sides of an imaginary line connecting the plain-line bonded substituents (as in structures L or M).
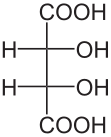
Another way to present stereo-centers is with help of Fischer projections which are designed not to employ any wedged lines. In this class we will not cover Fischer projections. The convention used is that all vertical bonds are pointing in, away from the viewer, and all horizontal bonds are pointing out, toward the viewer. Such structures can only have vertical and horizontal bonds on stereocenters, and cannot be rotated by 90° (without a change in stereochemistry). Often, the most oxidized carbon is placed at the top (but other arrangements are acceptable as well). Structure P (above) is the Fischer projection, and it could be translated into a "wedge" structure H (or structures L or M, above). One has to be careful to distinguish Fischer projections, such as P, from structures where no stereochemistry is explicitly shown, i.e. only plain bonds are used (in such cases 90° angles between drawn bonds should be avoided; see also below).
Hydrogen atoms attached to the stereocenter are often not shown explicitly, as is common for skeletal structures in general. If done carefully, the omission does not lead to complications and the stereo information is perfectly readable, but there are instances where ambiguity or loss of stereo information may occur. In general, at the learning stage, it is advisable to show stereocenter hydrogens explicitly.
These structures, labeled with the same letters as their originals above, illustrate clear and unambiguous examples of hydrogens omitted without any loss of stereochemical information. Structure P' is fine as long as it is made clear that it is a Fischer projection. On the other hand, other presentations may be questionable. For example, structure S can be interpreted (correctly) as structure L with hydrogen omitted, but (especially) when drawn by hand it may be interpreted as a poorly drawn structure of R, leading to the opposite 3D information. Despite appearances, structure T does not carry any stereochemical information (the position of hydrogen with its plain-line bond needs to be explicitly specified).
If stereochemistry is unspecified, no wedge lines are used, as in structure U. To easily distinguish such situation from Fischer projections, multiple 90° and 180° angles between drawn bonds should be avoided. Alternatively, situations when the stereochemistry is unknown, or a mixture of both enantiomers is present, can be indicated explicitly by a wavy line ( ) as shown in structure W. Similarly, the unspecified stereochemistry of the double bond (E or Z) may be shown by drawing "extended" formulas as in structure X. The wavy line in structure Y is an alternative way to indicate unknown stereochemistry or a mixture of isomers.
) as shown in structure W. Similarly, the unspecified stereochemistry of the double bond (E or Z) may be shown by drawing "extended" formulas as in structure X. The wavy line in structure Y is an alternative way to indicate unknown stereochemistry or a mixture of isomers.

http://en.wikipedia.org/wiki/Stereochemistry
(http://www.organicchemistryreview.com/STEREOCHEMISTRY.html)
Stereochemistry is also known as 3D chemistry because the prefix "stereo-" means "three-dimensionality".
The study of stereochemical problems spans the entire range of organic, inorganic, biological, physical and supramolecular chemistries. Stereochemistry includes methods for determining and describing these relationships; the effect on the physical or biological properties these relationships impart upon the molecules in question, and the manner in which these relationships influence the reactivity of the molecules in question (dynamic stereochemistry).
DRAWING STEREOCHEMICAL STRUCTURES FROM A TO Z
Bonds to the other two substituents are shown with different symbols. Bonds to atoms above the plane of the drawing (coming out, toward the viewer) are shown with a bold wedge (
Bonds to atoms below the plane (going in, away from the viewer) are shown with hash wedges (
These various presentations are illustrated below on an example of a compound with one stereocenter (in all cases, except where indicated, the same S absolute stereochemistry is shown). The first structure (A) is the favored presentation, while B shows a rotational variant of A (any rotation within the plane of these drawings is perfectly acceptable). Structure C demonstrates the European convention that has a more consistent perspective view of the wedges. Structure D shows the use of open wedge (not recommended), while structure E illustrates the use of bar bonds, that are commonly employed to designate relative stereochemistry. In our class we will use wedges (A) to indicate absolute stereochemistry (one enantiomer), and bars (E) to designate racemic mixtures (and relative stereochemistry, see below).
In addition to structures with two bonds to the stereocenter in the plane of the drawing (above), some representations may have only one bond in that plane (F and G) and some have none (H and I), as shown below. In structures like F and G or H and I, three or four wedged lines are used to designate the 3D disposition of substituents, respectively.
Confusingly, one may often encounter other 3D structures that seem to follow the above conventions, but are in fact different perspective representations. In structures J–M the plain bonds (–––) are not in the plane of the drawing. Their 3D disposition is implied by the wedge lines present. For example, in structure J the wedge bond is a mast pole on three legs, and in L and M the plain-line bonds are really going in or out of the plane of the drawing, respectively..
All these arrangements may be visualized in 3D by selecting the appropriate radio-button in the Jmol applet. Of course C, D, and E are in the exact same orientation as A and are not repeated. Note that all the manipulations are just simple rotations of the same molecule.
Paying attention to the correct representation of the perspective is crucial. Some incorrect usage of wedge bonds is illustrated in structures N and O. Despite appearances, there is no stereochemical information in such structures. A couple of simple rules can be used to decide adequacy of such drawings: (1) the bold-wedge and the hash-wedge substituents must be on the same side of an imaginary line connecting the plain-line bonded substituents (as in structures A or B), and (2) two bold-wedge subsituents (or two hash-wedge substituents) must be on the opposite sides of an imaginary line connecting the plain-line bonded substituents (as in structures L or M).
 |  |
Hydrogen atoms attached to the stereocenter are often not shown explicitly, as is common for skeletal structures in general. If done carefully, the omission does not lead to complications and the stereo information is perfectly readable, but there are instances where ambiguity or loss of stereo information may occur. In general, at the learning stage, it is advisable to show stereocenter hydrogens explicitly.
 |  |
If stereochemistry is unspecified, no wedge lines are used, as in structure U. To easily distinguish such situation from Fischer projections, multiple 90° and 180° angles between drawn bonds should be avoided. Alternatively, situations when the stereochemistry is unknown, or a mixture of both enantiomers is present, can be indicated explicitly by a wavy line (

For molecules with multiple stereocenters other methods of display are also available. The Newman projection in Z(1) unambiguously defines the absolute stereochemistry on both centers (S,S). Occasionally, a perspective drawing without any wedge or bar bonds is employed as shown in Z(2). This sawhorse representation is a "stretched" version of the Newman projection. When wedges or bars are used, the plain-line bonds should be used to connect the centers, as illustrated for structure Z(3). This approach avoids possible ambiguities as to which wedge bond belongs to which center.
As illustrated by structures Z(4) 'and Z(5) omitting stereocenter's hydrogens (if done properly) often increases the clarity of the presentation. Structure Z(4) with wedge bonds designate absolute stereochemistry, i.e. enantiomerically pure compound with two S centers. In our class, structure Z(5) with bars designates only the relative stereochemistry, i.e. it corresponds to the racemic mixture of (S,S) and (R,R) enantiomers.http://en.wikipedia.org/wiki/Stereochemistry
(http://www.organicchemistryreview.com/STEREOCHEMISTRY.html)